_____________________________________________________________________________
Za prijavu se javiti gđi. Petri Lazić na plazic@sfzg.hr

______________________________________________________________________
Poštovani studenti Stomatološkog fakulteta,
U sklopu provedbe projekta Internacionalizacije visokog obrazovanja, Vaš Fakultet ove godine, u suradnji s Medicinskim fakultetom, organizira međunarodnu ljetnu školu „Methods in Dental and Orofacial Tissues Research“.
Po prvi ćete puta imati priliku tijekom 5 dana intenzivno pohađati praktične radionice na kojima ćete se upoznati s metodama temeljnih znanstvenih istraživanja u području orofacijalnih znanosti. Uz nastavnike našeg Sveučilišta, za Vas u Zagreb dolaze znastvenici i nastavnici iz SAD-a, Londona, Zuericha i Singapura, od kojih su neki bivši studenti našeg fakulteta. Svi su međunarodno afirmirani znanstvenici, a u ovu aktivnost investiraju ogroman entuzijazam.
Ljetna škola je besplatna za sve polaznike. Po uspješnom završetku Ljetne škole, bit će Vam dodijeljeno 2 ECTS boda koje ćete moći koristiti sljedeće akademske godine kao zamjenu za izborni predmet.
Svi ste dobro došli, uz napomenu da smo načelno za Vas predvidjeli 10 mjesta. Uz Vas će biti polaznici iz Europe, što će Vam biti odlično iskustvo i prilika za umrežavanje.
Mjesto održavanja: Hrvatski institut za istraživanje mozga (Sveučilište u Zagrebu, Medicinski fakultet), Šalata 12
Vrijeme održavanja: 1-5. srpnja 2019. Početak je ponedjeljak 1.7. u 8 sati, a točan raspored će ovisiti o rasporedu grupa. Završetak je u petak 5.7. u 15 sati. Računajte na cjelodnevni program. Jezik održavanja je engleski.
Odazovite se i podržite ovaj inovativni koncept nastave koji za cilj ima uvesti Vas i cijelu našu struku u svijet modernih temeljnih istraživanja te pronađite znanstvenika u sebi!
Radujemo se zajedničkom druženju!
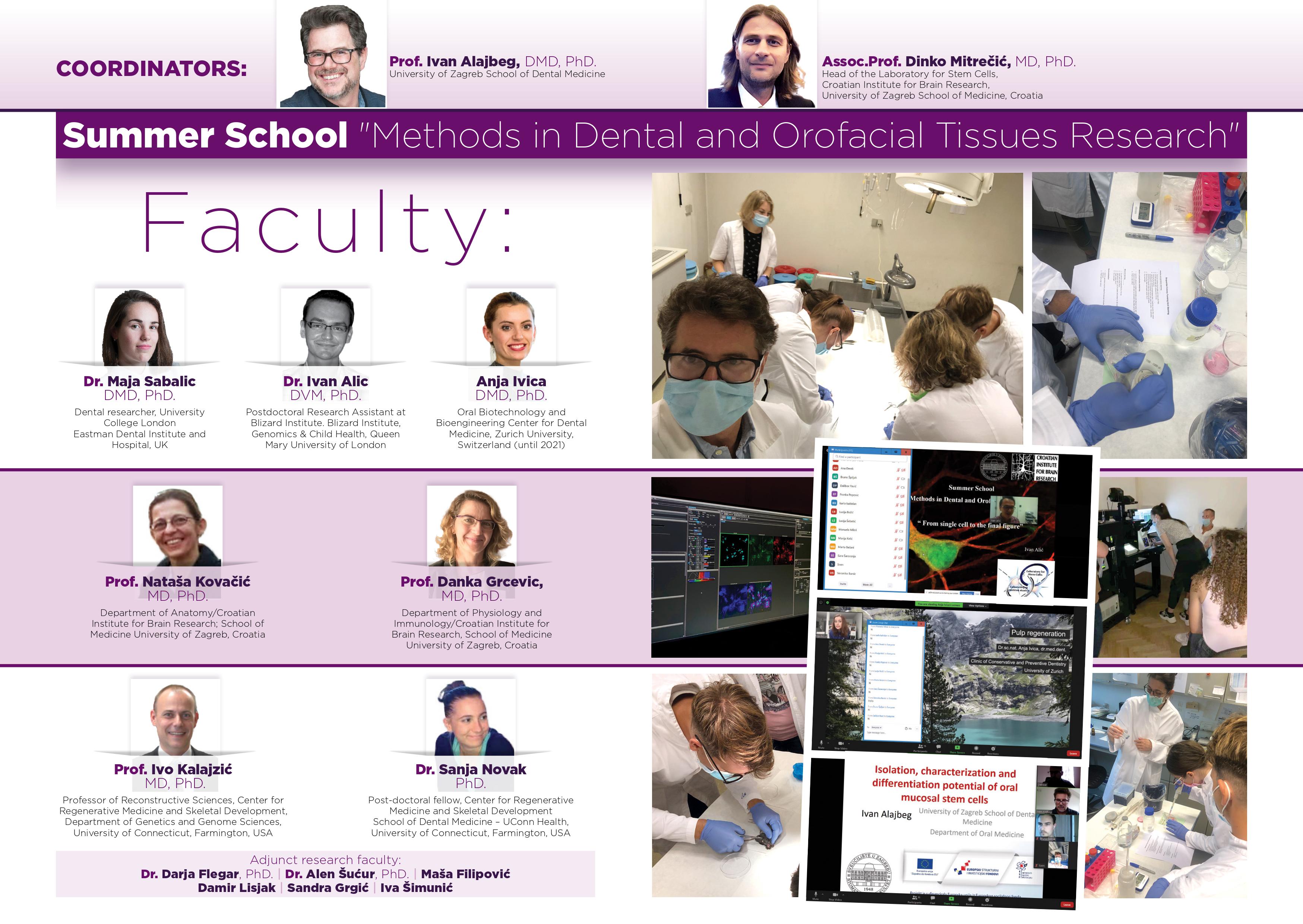
Faculty:
Professor Ivan Alajbeg, DMD, PhD; Department of Oral Medicine, University of Zagreb School of Dental Medicine
Assoc. Professor Dinko Mitrečić, MD, PhD; Head of the Laboratory for Stem Cells, Croatian Institute for Brain Research, University of Zagreb School of Medicine
Professor Danka Grčević, MD, PhD; Department of Physiology and Immunology/Croatian Institute for Brain Research, University of Zagreb School of Medicine
Assoc. Professor Nataša Kovačić, MD, PhD; Department of Anatomy/Croatian Institute for Brain Research, University of Zagreb School of Medicine
Professor Ivo Kalajzić, MD, PhD; Center for Regenerative Medicine and Skeletal Development, Department of Genetics and Genome Sciences, University of Connecticut, Farmington, USA
Dr. Sanja Novak, PhD; Center for Regenerative Medicine and Skeletal Development, Department of Genetics and Genome Sciences, University of Connecticut, Farmington, USA
Dr. Maja Sabalić Schoener, DMD, PhD; Eastman Dental Institute, Univ. College London, UK
Dr. Ivan Alić, DVM, PhD; Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore and University of Zagreb School of Veterinary Medicine
Dr. Anja Ivica, DMD. Oral Biotechnology and Bioengineering Center for Dental Medicine, Zurich University, Switzerland
Monday July 1:
Module 1
08:00 – 08:45 Introductory seminar
08:45 – onwards Work in Stem Cell Culture Laboratory (divided in lesser groups, 7 hours of program per group)
Students will get to know two different cell populations (oral lamina propria stem cells and pluripotent fibroblasts/mesenchimal mucosal cells) and compare with neural stem cells. They will change cell growth medium, perform enzymatic dissociation of cells, resuspending and seeding cells in Evos live cell imaging system.
Tuesday and Wednesday July 2 and 3
Roster of students in groups per modules during 2 days, both modules to be held both days
Module 2
08:00 – 08:45 Introductory seminar
08:45 – 12:30 Work in Stem Cell Culture Laboratory and MACS in regular laboratory
13:00 – onwards Bioinformatic workshop in Computer Lecture Room
Part 1. In the first practical, participants will have a hands-on experience of isolating cells for single-cell RNA sequencing, involving murine dental pulp dissections, enzymatic dissociation, removal of dead cells from the single-cell suspension using magnetic-activated cell sorting, cell counting using hemocytometer and calculating cell viability.
Part 2. In the second practical, participants will perform basic analysis and exploration of a publicly-available scRNA-seq dataset using web-based bioinformatic tools.
Module 3
08:00 – 08:45 Introductory seminar
08:45 – 12:30 Work in Stem Cell Culture Laboratory, in regular laboratory and in Computer Lecture Room
13:00 – onwards Work in Stem Cell Culture Laboratory, in regular laboratory and in Computer Lecture Room
Part 1: Cell migration towards differently pre-treated dentin disks. Each student will get 24-well plate with Transwell inserts. The day prior the experiment, cells would be seeded on the top of the insert and differently treated dentin disks would be placed in the well under the insert. Students will remove the medium from the inserts and wells, wash the inserts with PBS, fix cells (4% formaldehyde) and permeabilize them with methanol, stain the cells using Crystal violet and remove the cells from the upper part of the inserts using cotton swabs. After all, students will observe migrated cells on the bottom of the inserts, take pictures and count them using program ImageJ on their computers.
Part 2: Cell attachment. Students will disinfect dentin disks with NaOCl and treat them with different conditioning solution. After washing with PBS, the disks are ready for the next step.
Meanwhile, the students have to prepare cells. They will get T75 flask with the cells and the medium. They have to remove the medium, wash the cells with PBS to get rid of all the medium, detach the cells using Trypsin, add the new medium with serum to neutralize Trypsin, centrifuge the cells and resuspend the pallet. They have to count the cell density and calculate how much medium to add. This cell suspension is going to be used for cell seeding.
Students will apply the same amount of cell suspension on the top of the prepared dentin disks and leave them in the incubator for 4h. After incubation, the disks will be washed, cells would be detached and counted. The numbers represent the cells that managed to attach to the dentin surface during incubation time and results between different groups would be compared and discussed.
Thursday July 4
Module 4
08:00 – 08:45 Introductory seminar
08:45 – 12:30 and 13:00 – onwards: Work in Laboratory for Mollecular Immunology (Flow cytometry and Cryocut facilities) and in Facility for operative procedures on laboratory animals
Students will perform: tooth extraction (mouse molars); preparation of periodontal ligament (PDL) cells digestion for cell culture, preparation of cells for Flow cytometry, and labelling using antibodies, preparing sections for imaging (section attachment), and fluorescent imaging.
Friday July 5
Modules 1-4 recap
08:00 – 08:45 Module 1 recap and results– Seminar room and Labs
08:45 – 10:15 Module 2 and 3 recap and results - Seminar room and Labs
10:15 – 11:45 Module 4 recap and results- Seminar room and Labs
11:45 – 12:30 Final remarks and discussion – Seminar room
13:30 – 14.30 Test for ECTS credits

 Pristupačnost
Pristupačnost